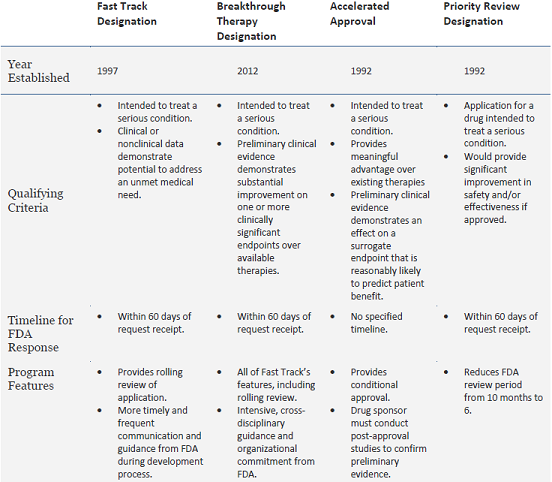
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts
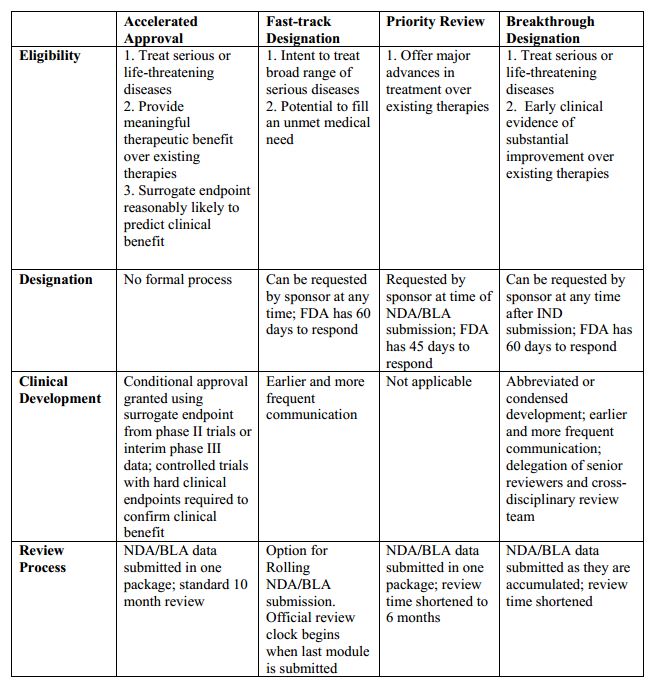
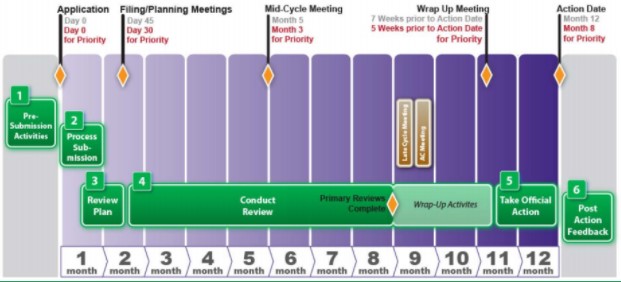
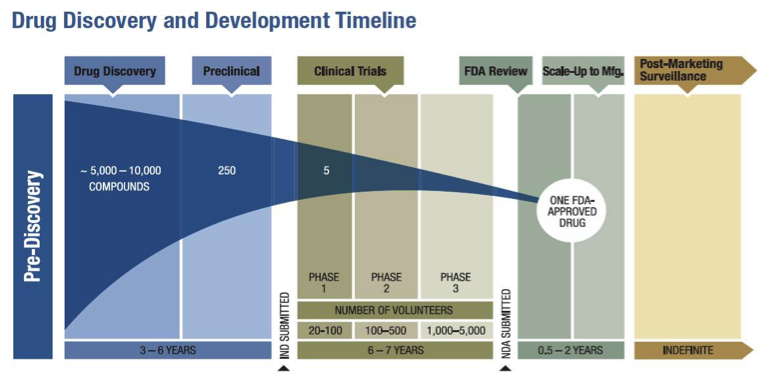
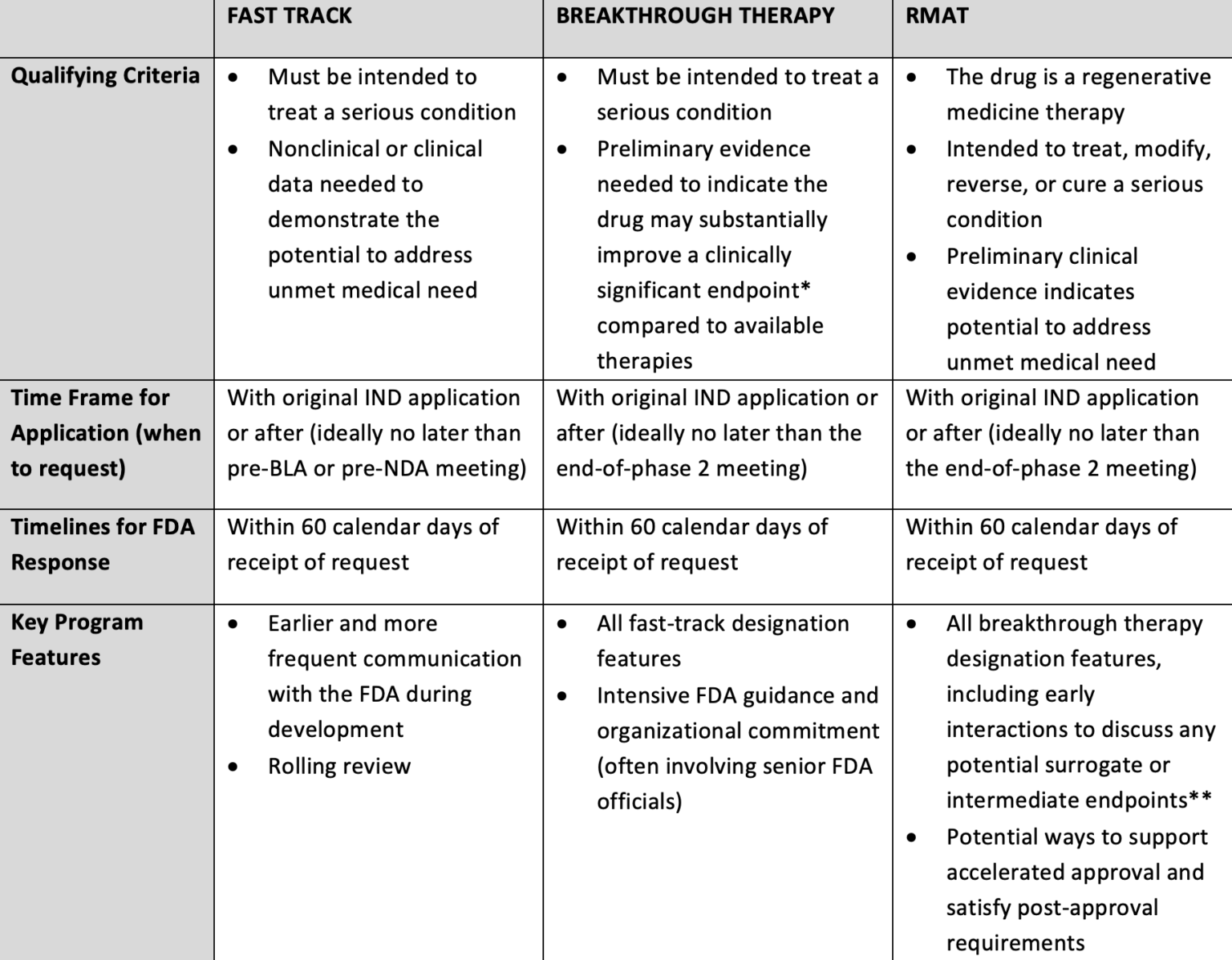
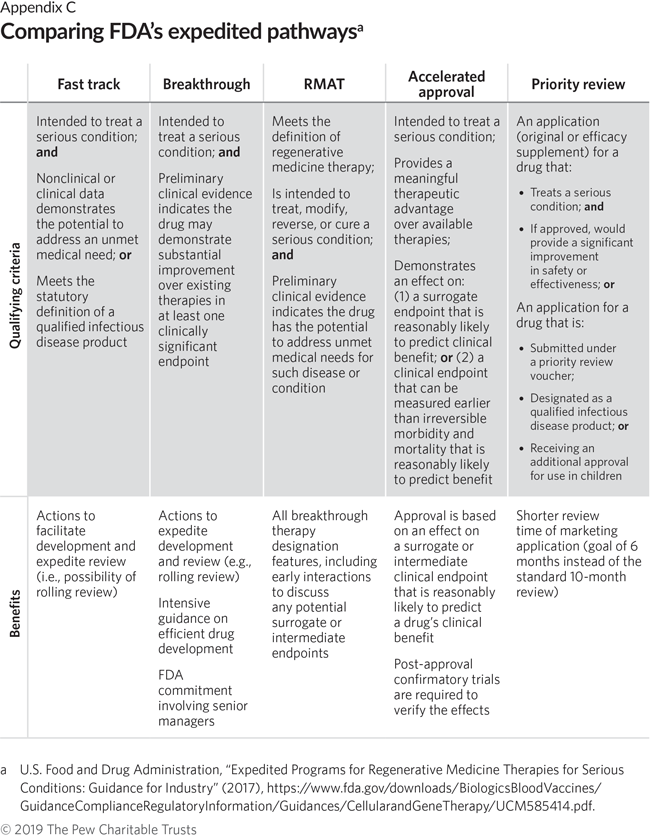
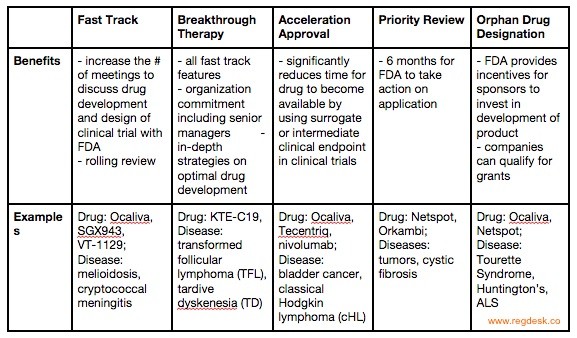
Making Sense of FDA's Expedited Drug Approval Pathways and Designations – for the Non-Regulatory Professional

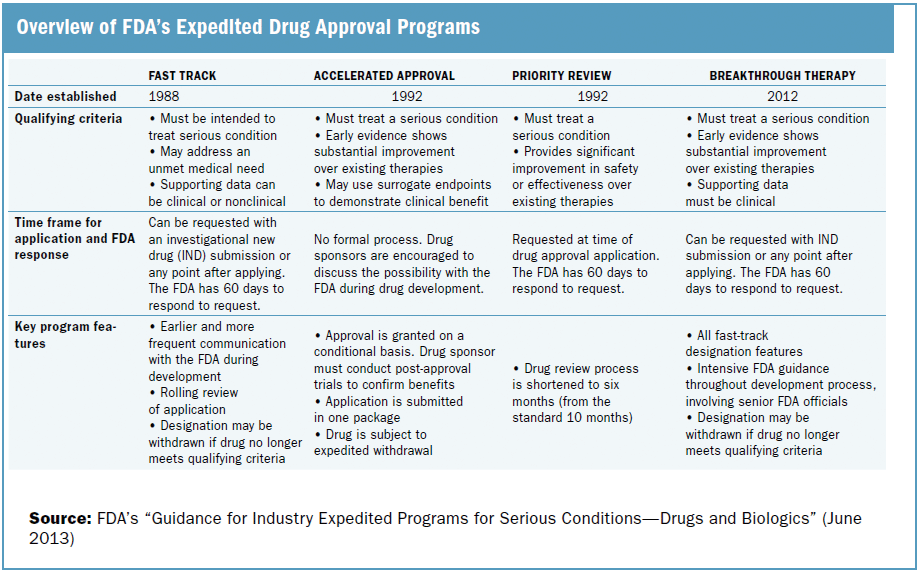
Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink

Y-mAbs Reports Initiation of Rolling Review of BLA for Naxitamab to the US FDA to Treat Neuroblastoma
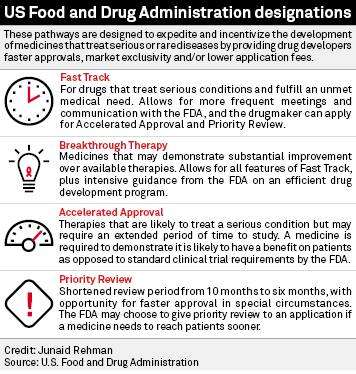
FDA Moves Against Fast Track Vaccines - News about Energy Storage, Batteries, Climate Change and the Environment

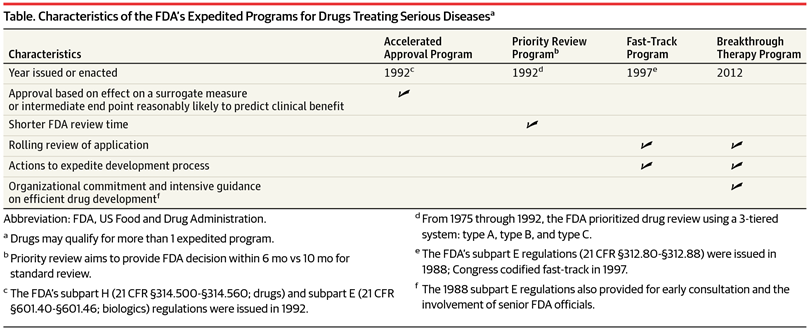
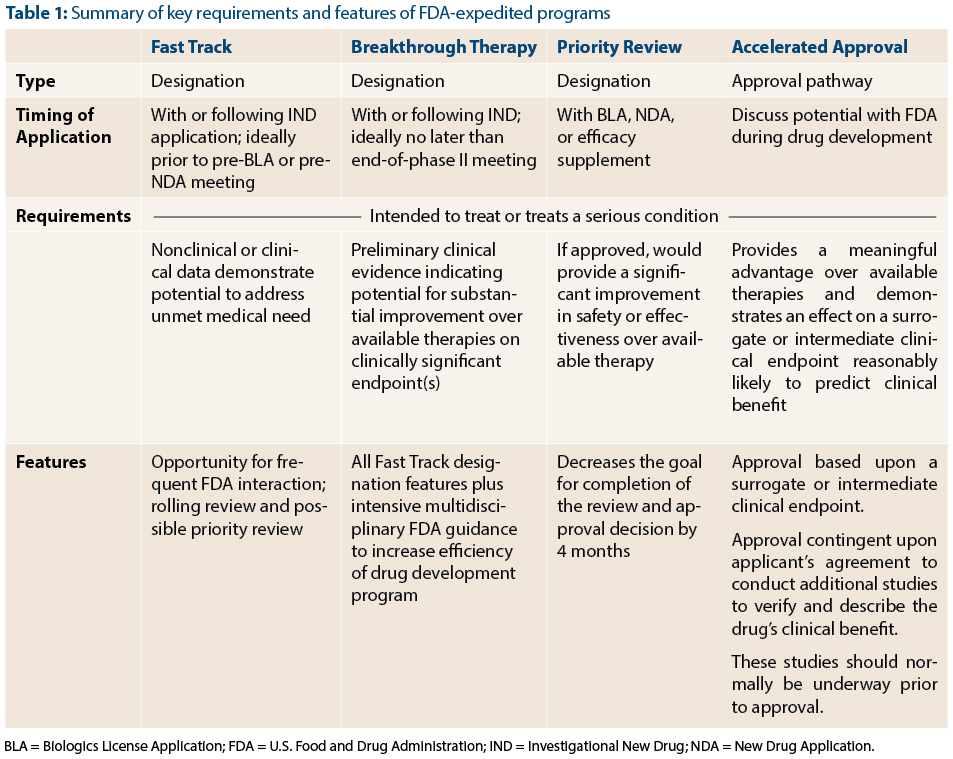
Update to Drugs, Devices, and the FDA: How Recent Legislative Changes Have Impacted Approval of New Therapies - ScienceDirect

Vertex/CRISPR to begin rolling review in US for gene-edited therapy; get FDA designations | Seeking Alpha

Nisarg Patel on Twitter: "Yet, we have a multitude of regulatory pathways to accelerate the development and review of COVID19 therapeutics without compromising clinical evidence. Here, we adopt elements of the Real-Time



/cloudfront-us-east-2.images.arcpublishing.com/reuters/LUSOBJZ5H5OIDD7VBNDDQP62F4.jpg)