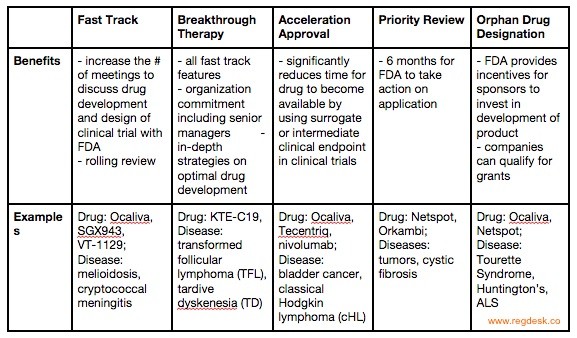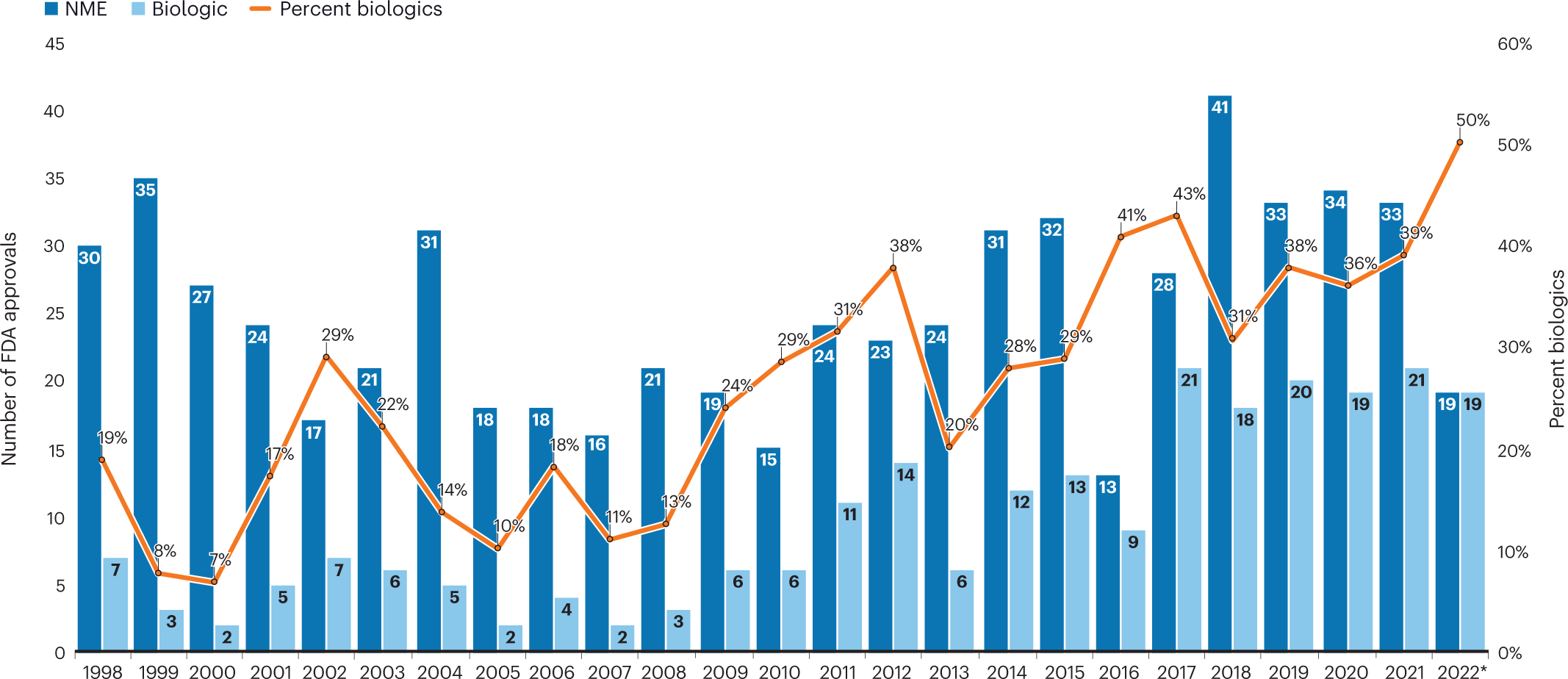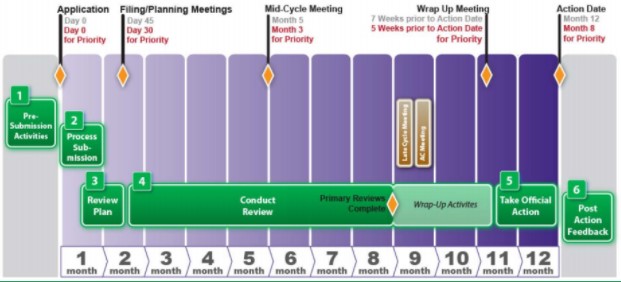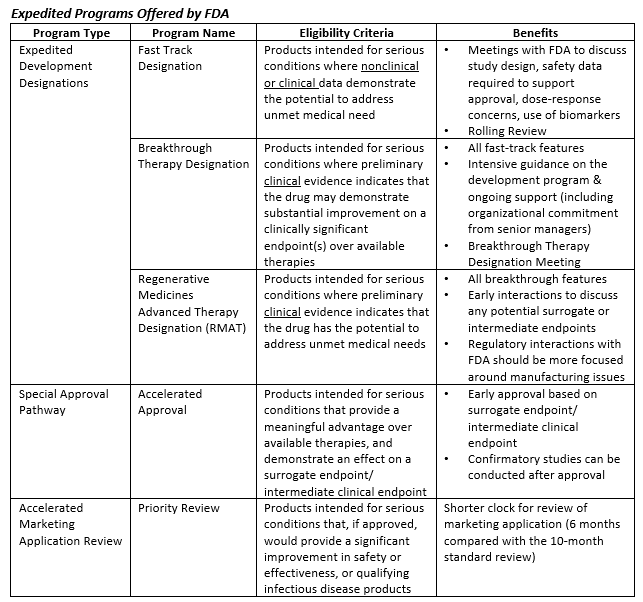
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group
Development and Regulation of Medical Countermeasures for COVID-19 (Vaccines, Diagnostics, and Treatments): Frequently Asked Questions - EveryCRSReport.com

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations
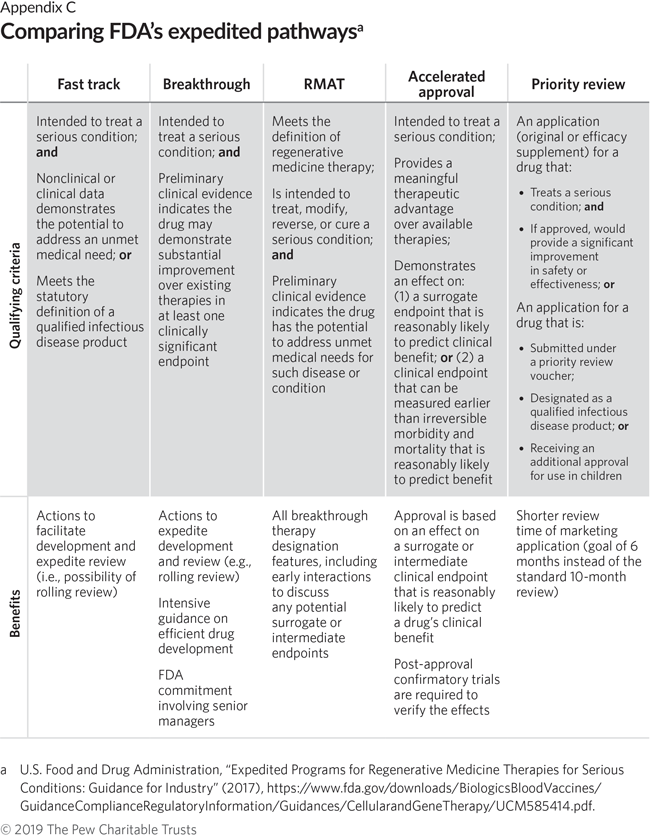
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts

Vertex/CRISPR to begin rolling review in US for gene-edited therapy; get FDA designations | Seeking Alpha

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink